Taking a new medical device from a bar-napkin drawing to the Operating Room is an expensive and time-consuming journey. Medical companies spend millions of dollars and years designing, building, and testing new surgical devices. The NRE (non-recurring engineering) associated with these costly developments is amortized over the anticipated income stream from sales of both the base instrument and the associated disposables. While the disposable handpieces associated with electrosurgery may seem like simple electro-mechanical devices once they are mass produced, their pricing includes a share of the original NRE. This provides an opportunity for counterfeiters to produce lower cost alternatives that do not meet the same standards as the original equipment manufacturer.
Taking a new medical device from a bar-napkin drawing to the Operating Room is an expensive and time-consuming journey. Medical companies spend millions of dollars and years designing, building, and testing new surgical devices. The NRE (non-recurring engineering) associated with these costly developments is amortized over the anticipated income stream from sales of both the base instrument and the associated disposables. While the disposable handpieces associated with electrosurgery may seem like simple electro-mechanical devices once they are mass produced, their pricing includes a share of the original NRE. This provides an opportunity for counterfeiters to produce lower cost alternatives that do not meet the same standards as the original equipment manufacturer.
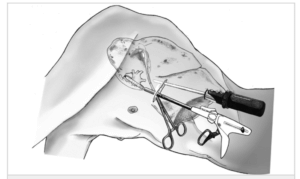 Electrosurgical devices are used for minimally invasive or open surgical procedures where ligation and division of vessels, tissue bundles, and lymphatics is desired. The device allows the surgeon to grasp, cut, dissect, and reliably seal tissue and vessels. Disposable electrosurgical handpieces have a high margin, and as a result they are routinely reverse engineered by counterfeiters, thereby flooding the marketplace with cheap knockoffs. These counterfeit devices significantly increase the surgical risk to the patient and damage the reputation and profitability of the original medical manufacturer.
Electrosurgical devices are used for minimally invasive or open surgical procedures where ligation and division of vessels, tissue bundles, and lymphatics is desired. The device allows the surgeon to grasp, cut, dissect, and reliably seal tissue and vessels. Disposable electrosurgical handpieces have a high margin, and as a result they are routinely reverse engineered by counterfeiters, thereby flooding the marketplace with cheap knockoffs. These counterfeit devices significantly increase the surgical risk to the patient and damage the reputation and profitability of the original medical manufacturer.
Using RFID to Prevent Counterfeit Medical Devices
A major medical company that designs and manufacturers electrosurgical devices engaged with Cardinal Peak to evaluate possible solutions to the counterfeiting problem. A method had to be devised to make the disposable handheld instruments difficult, if not impossible, to counterfeit without compromising the high quality of the device. This anti-counterfeiting solution had to be cost-effective and add no additional burden to the hospital staff. After multiple solutions were investigated, the decision was made to add an RFID chip to each procedure-specific handpiece. This would require a redesign of all the handpieces in the product line and modification of the software in the electrosurgical energy platform.
By integrating RFID technology, Cardinal Peak helped reduce the risk of counterfeit electrosurgical handpieces, safeguarding patient health and enhancing surgical safety.
 Our client began a multi-year effort to redesign each handpiece while Cardinal Peak was asked to architect the software solution and implement the new anti-counterfeiting feature in the existing electrosurgical energy platform. The client managed the project in accordance with their Quality Management System (QMS) and Standard Operating Procedures (SOP). Cardinal Peak software developers were grounded in the appropriate QMS procedures and SOPs, thus providing a talented and trained team ready to participate in the effort for the duration of the project.
Our client began a multi-year effort to redesign each handpiece while Cardinal Peak was asked to architect the software solution and implement the new anti-counterfeiting feature in the existing electrosurgical energy platform. The client managed the project in accordance with their Quality Management System (QMS) and Standard Operating Procedures (SOP). Cardinal Peak software developers were grounded in the appropriate QMS procedures and SOPs, thus providing a talented and trained team ready to participate in the effort for the duration of the project.
The new anti-counterfeiting software feature had to provide a means to read the RFID chip in the handpiece and verify that it was a genuine device, manufactured by the medical company. It also needed to read additional information about the handpiece to ensure the device would be used correctly in the surgical procedure and not inappropriately reused on multiple patients or used beyond its intended lifetime.
The Cardinal Peak software team designed and implemented the new software features to execute on an existing embedded Linux platform, writing the object-oriented code in C/C++. FMEA (Failure Mode and Effects Analysis) was performed throughout the design process to identify and mitigate risks. In-depth code reviews and project audits were held. Required documentation was written. Finally, lab testing was performed to ensure correct operation of the modified handpieces with the updated energy platform.
The product development process conformed to the appropriate standards, including: 13485, 62304, 14971, etc.
Electrosurgical Medical Device Challenges
Some of the more challenging aspects of the software design and implementation included:
- Envisioning and guarding against possible competitive responses to the new RFID solution
- Inclusion of a cryptographic signature over the RFID and messaging to further protect against counterfeiting
- Embedding of new feature code into an existing product and conducting regression testing to ensure all features work as intended
- Accommodating a wide variety of handpiece/platform combinations during lab debugging and functional testing
- Providing extensive documentation to support the project hand-off
By implementing software to combat counterfeit handpieces in the marketplace, Cardinal Peak helped our medical client safeguard patient health by reducing the risk of surgical anomalies. We also protected our client’s reputation and profitability by ensuring only genuine handpieces can be used with the client’s energy platform. Anticounterfeiting measures is another way Cardinal peak adds value to the medical products in the marketplace.
If you are looking for an expert medical device development partner, reach out to discuss your project and get accurate estimates for budget and timeline.
Check out our related blog post on the role of near-field communication (NFC) in counterfeit protection.